skip to main |
skip to sidebar
Objectives
- Construct models of molecules
- Determine molecular shapes
- Predict polarity of molecules
Materials
Procedure
- We built a model for each of the listed molecules on the back of our given paper.
| CH4 |
BF3 |
C3H8 |
H2O |
Si2H6 |
HF |
CH3NH2 |
H2O2 |
N2 |
SeF4 |
C2H4 |
SiH2O |
IF3 |
SF6 | CO2 |
SO32 |
- Drew the three-dimensional structure for each of these molecules
- Then determine each of the molecules; shape, bond angle, polarity, and resonance
Results
(We're sorry - we've tried to fix the blank space, but because of the limited amount of space blogger layouts give us, we can't. Please scroll down. I know it's a hassle, but it's worth it when you get to the bottom. Thanks)

| Molecular Formula |
Lewis Structure |
3-Dimensional Model |
Shape |
Bond Angle |
Polarity |
Resonance |
CH4 |
 | row 1, cell 3 |
tetrahedral |
109° |
no |
no |
BF3 |
 | row 2, cell 3 |
triagular planar
|
120° |
yes |
no |
C3H8 |
 |  | linear | 120° |
no |
no |
|
H2O |
 |  | angular | 109.5° | |
yes
|
no |
|
Si2H6 |
 |  |
|
108.9° |
no |
no |
HF |
 |  |
linear |
180° |
yes |
no |
CH3NH2 |
 |
row 7, cell 3 |
tetrahedral |
120° |
yes |
no |
H2O2 |
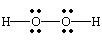 | row 8, cell
|
|
row 8, cell 4
|
row 8, cell 5 |
no |
no |
N2 |
 |
row 9, cell 3 |
linear |
180° |
no |
no |
SeF4 |
 |  |
seesaw |
row 10, cell 5 |
no |
no |
C2H4 |  |  |
planar |
120° |
no |
no |
SiH2O |
 |
row 12, cell 3 |
trigonal planar
|
120° |
yes |
no |
IF3 |
 |  |
T-shaped |
180° |
yes |
no |
SF6 |
 |  |
tetrahedral
|
180° |
yes |
no |
CO2 |
 |  |
linear |
180° |
no |
no |
SO32- |
 |
 |
trigonal planar
|
104.5° |
row 16, cell 6 |
yes
|
Paper Chromatography Lab
- We were trying to figure out which solvent out of the four chosen (H2O, CH3OH, C3H7OH, and C6H14) would best work to mobilize the components (water-based markers).
Hypothesis
- We hypothesized that the C3H7OH would be the best solution and move the component farther due to the amount of Hydrogen atoms.
Materials
- H2O
- CH3OH
- C3H7OH
- C6H14
- Black, Red, Green, Purple, and Blue Overhead Pens
- Chromatography Paper (Cut into 1 cm by 8 cm strips)
Safety Precautions
- Goggles
- Aprons
- To avoid contact with the Hexane we prepped our station before hand and made sure to keep the goggles and aprons fully covering their designated areas. We made extra sure to keep the Hexane in use under the fume hood and to keep our heads away from the fumes.
Procedure
- In Part I of the Chromatography Lab we took four pieces of the Chromatography paper and dotted them three times with a black overhead pen. We then took our well pan to the chemical dispensary fume hood to retrieve the different solvents to use in our experiment. With the caution of the Hexane in mind we took the well pan directly back to our stations' fume hood. We separately labeled the chromatography papers for which solvents they would be placed into and then we put the papers into the separate solvents. After waiting a half an hour we observed the papers with the following results. In order from left to right is H2O, CH3OH, C3H7OH, and C6H14.

- In Part II of the Chromatography Lab we choose a solvent which worked best determined in Part I of the lab. (H2O!) We then took various colors of overhead pens such as Red, Green, Purple, and Blue. Dotted separate chromatography papers for each pen color, then set them in the well plate, already filled with H2O. We got the following results in the following order; Blue, Purple, Red, and Green

Conclusion
- The results of our experiment show that our hypothesis that C3H7OH would work best due to the amount of Hydrogen atoms was incorrect and therefore thrown out.
- H2O worked the best out of the four given solvents, because of it's polarity.
- CH3OH worked second best
- C3H7OH came in third
- And C6H14 worked the worst.
Learning Experience
- Through this experimental lab we learned that safety precautions are very important when dealing with toxic chemicals. Thus keeping a pair of goggles and an apron on was necessary at all times.
Technical Difficulties
- Any experiment could face difficulties. Our experiment could have been better with a wider range of colors and solvents. We could have compared different solvents to see if we could find one more polar then H2O. This could have immensely improved our experiment.





























