skip to main |
skip to sidebar
Polarity and Molecular Shape Lab
Objectives
- Construct models of molecules
- Determine molecular shapes
- Predict polarity of molecules
Materials
Procedure
- We built a model for each of the listed molecules on the back of our given paper.
| CH4 |
BF3 |
C3H8 |
H2O |
Si2H6 |
HF |
CH3NH2 |
H2O2 |
N2 |
SeF4 |
C2H4 |
SiH2O |
IF3 |
SF6 | CO2 |
SO32 |
- Drew the three-dimensional structure for each of these molecules
- Then determine each of the molecules; shape, bond angle, polarity, and resonance
Results
(We're sorry - we've tried to fix the blank space, but because of the limited amount of space blogger layouts give us, we can't. Please scroll down. I know it's a hassle, but it's worth it when you get to the bottom. Thanks)

| Molecular Formula |
Lewis Structure |
3-Dimensional Model |
Shape |
Bond Angle |
Polarity |
Resonance |
CH4 |
 | row 1, cell 3 |
tetrahedral |
109° |
no |
no |
BF3 |
 | row 2, cell 3 |
triagular planar
|
120° |
yes |
no |
C3H8 |
 |  | linear | 120° |
no |
no |
|
H2O |
 |  | angular | 109.5° | |
yes
|
no |
|
Si2H6 |
 |  |
|
108.9° |
no |
no |
HF |
 |  |
linear |
180° |
yes |
no |
CH3NH2 |
 |
row 7, cell 3 |
tetrahedral |
120° |
yes |
no |
H2O2 |
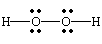 | row 8, cell
|
|
row 8, cell 4
|
row 8, cell 5 |
no |
no |
N2 |
 |
row 9, cell 3 |
linear |
180° |
no |
no |
SeF4 |
 |  |
seesaw |
row 10, cell 5 |
no |
no |
C2H4 |  |  |
planar |
120° |
no |
no |
SiH2O |
 |
row 12, cell 3 |
trigonal planar
|
120° |
yes |
no |
IF3 |
 |  |
T-shaped |
180° |
yes |
no |
SF6 |
 |  |
tetrahedral
|
180° |
yes |
no |
CO2 |
 |  |
linear |
180° |
no |
no |
SO32- |
 |
 |
trigonal planar
|
104.5° |
row 16, cell 6 |
yes
|




























WOW, awesome job guys! Id say just get rid of all the extra space between your information.
ReplyDeleteNice Lewis structures. Fill in the blanks with more words.
ReplyDeletebut good job(:
Nice job with the table. It is way more organized than ours and is easy to follow. But don't forget to fill it in all the way.
ReplyDeletehaha so imma agree with the other comments
ReplyDelete((delete the unused space))
and it would be nice to see more match up pictures
but great job guys looks fabulous!
The gaps make it difficult to follow but the way you have the pics arranged is great. :D
ReplyDeleteNice, but why is there so much distance between each section.
ReplyDeleteagain i agree delete unused space..
ReplyDeleteExcellent organization! Easy to follow and easy to read. :~)
ReplyDeleteOH MY GOODNESS! That's neat. I wish I could achieve that. Thanks for the note also.
ReplyDelete